By Kevin E. Noonan —
 The avocado, having gained popularity (at least in the U.S.) as a convenient (and delicious) vehicle for consuming otherwise not particularly healthful corn chips, has more recently been hailed as a "superfood" when consumed in diets as a healthy form of fat. Recently, the avocado joined the ranks of other plants important for human nutrition (see, for example, "Genomic Sequence of Strawberry Determined"; "Genetic Analyses of Sweet Potato Genome Sheds Light on Speciation and Global Dispersion Patterns"; "Durum Wheat Genome Revealed"; and "Genetic Assessment of Squash Genomes in Related Species") in having the sequence of its genome determined, with concomitant explication of its evolutionary history and relationship to other members of the plant kingdom.
The avocado, having gained popularity (at least in the U.S.) as a convenient (and delicious) vehicle for consuming otherwise not particularly healthful corn chips, has more recently been hailed as a "superfood" when consumed in diets as a healthy form of fat. Recently, the avocado joined the ranks of other plants important for human nutrition (see, for example, "Genomic Sequence of Strawberry Determined"; "Genetic Analyses of Sweet Potato Genome Sheds Light on Speciation and Global Dispersion Patterns"; "Durum Wheat Genome Revealed"; and "Genetic Assessment of Squash Genomes in Related Species") in having the sequence of its genome determined, with concomitant explication of its evolutionary history and relationship to other members of the plant kingdom.
This achievement was reported in the Proceedings of the National Academy of Sciences USA, in a paper entitled "The avocado genome informs deep angiosperm phylogeny, highlights introgressive hybridization, and reveals pathogen-influenced gene space adaptation" (https://doi.org/10.1073/pnas.1822129116) by an international group of researchers.* The avocado, Persea americana, is a member of the Lauraceae family, otherwise known for the spices cinnamon, bay leaves, and sassafras (gumbo filé). Avocadoes anciently diverged from other angiosperms, and their magnoliid clade comprises 11,000 total species, which the authors assert is "minuscule in comparison to the dominant eudicot and monocot flowering plant lineages, comprising about 285,000 species combined." Conventional genetic analyses had not established the avocados' phylogenetic position relative to eudicots and monocots.
The predominant avocado is the Mexican avocado, P. americana var. drymifolia, comprising three varieties: the Mexican, Guatemalan, and West Indian varieties. Familiar to many afficianados is the Hass cultivar, which is a hybrid between the Guatemalan and Mexican races and is typically grafted onto Mexican race rootstock. Worldwide, 90% of cultivated avocado corresponds to the cultivar Hass; surprisingly, the Hass cultivar is of recent (20th century) origin.
The authors sequenced Hass and Mexican cultivars as reference genomes, and resequenced three other cutivars (vars. drymifolia, guatemalensis, and americana), as well as wild avocados of the West Indian variety (P. america1na var. costaricensis), and Persea shiedeana (the edible coyo), a species that is relatively closely related to P. americana. The avocado genome comprises 12 chromosomes, and these authors report that the genome size of P. americana var. drymifolia is 920 Mb in size, while the P. americana Hass cultivar has a 912.6 Mb estimated genome size. Analysis of protein coding sequences was based on a comparison with protein sequences from the following angiosperm species: Sorghum bicolor (33,032 proteins), Vitis vinifera (26,343 proteins), Solanum lycopersicum (34,727 proteins), and Arabidopsis thaliana (27,416 proteins). These authors reported an estimated number of protein-coding genes in each genome: 22,441 protein-coding genes from the Mexican genome and 24,616 protein-coding genes from the Hass cultivar genome.
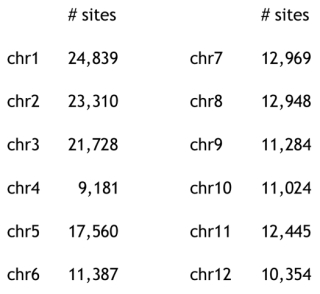
Turning to genome structure, the authors used principal component analysis of genome-wide single nucleotide polymorphisms (SNPs) to show relative uniformity in Costa Rican/West Indian/Guatemalan cultivars but strong heterogeneity within the Mexican subpopulation. The paper reported 5,050 SNP markers, distributed as follows:
 Results of these analyses of the Hass cultivar were intermediate between the Guatemalan and Mexican subpopulations, which was in agreement with the hybrid nature of this variety. Also consistent with the recent appearance of the Hass cultivar, its genome showed significant (39%) introgression of DNA having Guatamalan variety structure into a Mexican genomic background. Haas cultivar Chromosome 4 illustrates these analyses, demonstrating that a "huge" Guatemalan block, which could encompass an entire chromosomal arm, is present in the Hass genome. Figure 2A (at right) in the article illustrates a 22 megabase (Mb) stretch of DNA on chromosome 4 exemplifyng genomic introgression from the Guatemalan avocado race (var. guatemalensis) into a Mexican (var. drymifolia) genetic background.
Results of these analyses of the Hass cultivar were intermediate between the Guatemalan and Mexican subpopulations, which was in agreement with the hybrid nature of this variety. Also consistent with the recent appearance of the Hass cultivar, its genome showed significant (39%) introgression of DNA having Guatamalan variety structure into a Mexican genomic background. Haas cultivar Chromosome 4 illustrates these analyses, demonstrating that a "huge" Guatemalan block, which could encompass an entire chromosomal arm, is present in the Hass genome. Figure 2A (at right) in the article illustrates a 22 megabase (Mb) stretch of DNA on chromosome 4 exemplifyng genomic introgression from the Guatemalan avocado race (var. guatemalensis) into a Mexican (var. drymifolia) genetic background.
The authors further report that the relatively long length of this Guatemalan-derived block is uninterrupted by recombination, reflecting the extremely recent hybrid origin of the cultivar.
Further, genomic analysis revealed evidence of two "ancient" polyploidy events, which the authors report are lineage-specific and both postdate the divergence of the avocado from common ancestry with other angiosperm species. Avocado divergence from other species appears to have occurred from 7.4 to 3.8 million years ago. Unfortunately, these relationships could not be unambiguously resolved because the outcome varied with the way the analysis was performed. Based on comparison of protein sequences, for example, avocado was resolved as a sister to monocots plus eudicots (i.e., branching occurred before their divergence from each other), whereas from analysis of coding sequences avocado was placed as sister to monocots only.
The authors also reported the phenotypic effects of genome structure, specifically the occurrence of tandem duplications, on gene expression related to potentially important metabolic responses. Enrichment of tandem duplications was found in chromosomal regions that the authors contend could be related to recent adaptation against fungal pathogens. On the other hand, ancient polyploid duplicates were enriched with "transcriptional regulatory functions reflective of core physiological and developmental processes" (specifically, "among 2,433 total polyploid duplicates, regulation of transcription, DNA-templated was significantly overrepresented by 352 genes"). Transcriptional activity in tandem duplicates was found to increase following anthracnose infection, the authors positing that some of the up-regulated genes could be related to defense responses. These included genes involved in phenylpropanoid biosynthesis and closely related pathways that are significantly enriched among tandem duplicates. The authors further state:
This functional enrichment in a long-lived tree may have evolved in response to pathogen infection, including Colletotrichum (anthracnose) and Phytophthora cinnamomi (avocado root rot), both of which are reported to activate the phenylpropanoid biosynthetic pathways in avocado. . . . Several . . . functional enrichments among avocado tandems (for example, 1,3-beta-D-glucan synthase activity and regulation of cell shape) relate to callose synthase activity, a recently discovered avocado defense mechanism against P. cinnamomi. Other significantly enriched [metabolic pathways] include phenylpropanoid metabolic process, lignin biosynthetic process, and UDP-glycosyltransferase activity, categories directly or closely related to phenylpropanoid biosynthesis. The lignin functional enrichment, for example, includes diverse tandemly duplicated genes involved in many pathway-interrelated processes, including homologs of both biosynthetic and regulatory genes encoding hydroxycinnamoyl-CoA shikimate/quinate hydroxycinnamoyl transferase (HCT), cinnamyl alcohol dehydrogenase 5 (CAD5), laccase 17 (LAC17), caffeate o-methyltransferase 1 (COMT1), peroxidase 52 (PRX52), NAC domain containing protein 12 (NAC012), and NAC secondary wall thickening promoting factor 1 (NST1). As could be expected from the above, the [metabolic pathways] involved in defense response and defense response to fungus are significantly enriched among tandem duplicates, as has been discovered for other plant genomes, and involving many different gene families and responses. Tandem O-methyltransferases homologous to COMT1 may also contribute to synthesis of the phenylpropanoid derivative and insecticide estragole, which is largely responsible for the anise-like leaf scent and fruit taste of many avocado cultivars, particularly of the Mexican race. Another relevant enriched . . . category among tandems is ethylene-activated signaling pathway, which annotates many different transcription factor duplicates. Ethylene signaling factors such as ERF1 (represented by 2 homologs) are heavily involved in pathogen-induced responses, including to infection by Colletotrichum and other necrotrophic fungi. Also identified are 3 homologs of EIN3, a transcription factor that initiates downstream ethylene responses, including fruit ripening. Avocado fruit matures on the tree in a process that involves ethylene synthesis and signaling, while it does not ripen until harvested—a desirable trait that allows growers to delay harvesting for several months.
The authors characterize tandem duplicates as "evolutionarily recent 'tuning knobs' in the genome adaptive landscape[]" of the avocado species:
[M]ost tandem duplicates in the genome are expected to be of more recent origin, having been generated by ongoing gene birth–death–innovation processes that operate in all eukaryotic genomes. As such, sub- or neofunctionalized tandem duplicates that survive the usual fate of duplicated genes—pseudogenization—should be enriched in functions that fine-tune a given species' recent selective environment. In the case of avocado, response to fungal pathogens is precisely reflected in its tandemly duplicated gene complement.
The authors conclude their report with a concise explanation of its significance:
Our genomes of Mexican and Hass avocados provide the requisite resources for genome-wide association studies to identify important traits among natural avocado genetic diversity present in Mesoamerica, to develop genome-assisted breeding and genetic modification efforts crucial for the improvement of this long-life-cycle crop, to fight threatening avocado diseases, and to optimize growth and desirable phenotypic traits.
* Martha Rendón-Anayaa,b,1,
Enrique Ibarra-Laclettea,c,1,
Alfonso Méndez-Bravoa,d,
Tianying Lane,
Chunfang Zhengf,
Lorenzo Carretero-Pauletg,
Claudia Anahí Perez-Torresa,c,
Alejandra Chacón-Lópeza,
Gustavo Hernandez-Guzmána,h,i,
Tien-Hao Change,
Kimberly M. Farre,
Brad Barbazukj,
Srikar Chamalak,
Marek Mutwill,
Devendra Shivharel,
David Alvarez-Poncem,
Neena Mittern,
Alice Haywardn,
Stephen Fletchern,
Julio Rozaso,p,
Alejandro Sánchez Graciao,p,
David Kuhnq,
Alejandro F. Barrientos-Priegor,
Jarkko Salojärvil,
Pablo Librados,t,
David Sankofff,
Alfredo Herrera-Estrellaa,
Victor A. Alberte,l,2, and
Luis Herrera-Estrellaa,u,2
aUnidad de Genomica Avanzada/Langebio, Centro de Investigación y de Estudios Avanzados, Irapuato 36821, México; bDepartment of Plant Biology, Uppsala BioCenter, Swedish University of Agricultural Sciences, SE-750 07 Uppsala, Sweden; cRed de Estudios Moleculares Avanzados, Instituto de Ecología A.C., 91070 Xalapa, México; dEscuela Nacional de Estudios Superiores, Laboratorio Nacional de Análisis y Síntesis Ecológica, Universidad Nacional Autónoma de México, 58190 Morelia, México; eDepartment of Biological Sciences, University at Buffalo, Buffalo, NY 14260; fDepartment of Mathematics and Statistics, University of Ottawa, Ottawa, ON, Canada K1N 6N5; gCenter for Plant Systems Biology, Vlaams Instituut voor Biotechnologie (VIB), University of Ghent, 9052 Ghent, Belgium; hDepartamento de Alimentos, Universidad de Guanajuato, 36500 Irapuato, México; iDivisión de Ciencias de la Vida, Universidad de Guanajuato, 36500 Irapuato, México; jDepartment of Biology, University of Florida, Gainesville, FL 32611; kDepartment of Pathology, Immunology and Laboratory Medicine, University of Florida, Gainesville, FL 32610; lSchool of Biological Sciences, Nanyang Technological University, Singapore 637551; mDepartment of Biology, University of Nevada, Reno, NV 89557; nCentre for Horticultural Science, Queensland Alliance for Agriculture and Food Innovation, The University of Queensland, St. Lucia, QLD 4072, Australia; oDepartament de Genètica, Microbiologia i Estadística, Universitat de Barcelona, 08007 Barcelona, Spain; pInstitut de Recerca de la Biodiversitat, Universitat de Barcelona, 08007 Barcelona, Spain; qSubtropical Horticulture Research Station, Agricultural Research Service, US Department of Agriculture, Miami, FL 33158; rPosgrado en Horticultura, Departamento de Fitotecnia, Universidad Autónoma Chapingo, 56230 Texcoco, México; sCentre for GeoGenetics, Natural History Museum of Denmark, 1017 Copenhagen, Denmark; tLaboratoire d'Anthropobiologie Moléculaire et d'Imagerie de Synthèse, CNRS Unité Mixte de Recherche 5288, Université de Toulouse, Université Paul Sabatier, 31330 Toulouse, France; uDepartment of Plant and Soil Science, Texas Tech University, Lubbock, TX 79409
Image of Avocados (Persea americana) by B.navez, from the Wikimedia Commons under the Creative Commons Attribution-Share Alike 3.0 Unported, 2.5 Generic, 2.0 Generic and 1.0 Generic license.

Leave a comment