By Kevin E. Noonan –
 The "genome revolution" over the past 30 years has resulted in the elucidation of several species, both domesticated (see, e.g., "The Genetic Basis of Coat Variation in Dogs"; "Further and More Detailed Study of Domestic Cat Genome"; "Chicken Origins Established (But Philosophical Questions Remain)"; "Rose Genome Reveals Its Exquisite Complexities") and not ("Red Fox Genome Sheds Light on Domesticated Dogs (and Maybe Humans)"; "Lowland Gorilla Genome Sequenced"; "Giraffe Genome Reveals Relevant Adaptations"; "Genome Structure of the American Cockroach"). Recently, a group of researchers from the U.S., Canada, Europe, and the United Kingdom published the results of their studies with the blue whale, Balaenoptera musculus, which while being "the largest animal to have ever existed, reaching up to 110 feet in length and weighing 330,000 pounds" does not have the largest eukaryotic genome (at 2.7 billion base pairs (Gbps), compared with Paris japonica at 149 Gbps and the Australian lungfish at 43 Gbps); see Bukhman et al., 2024, A high-quality blue whale genome, 1 segmental duplications, and historical demography, Molec. Biol. Evol. Vol. 41, No. 3; https://doi.org/10.1093/molbev/msae036).
The "genome revolution" over the past 30 years has resulted in the elucidation of several species, both domesticated (see, e.g., "The Genetic Basis of Coat Variation in Dogs"; "Further and More Detailed Study of Domestic Cat Genome"; "Chicken Origins Established (But Philosophical Questions Remain)"; "Rose Genome Reveals Its Exquisite Complexities") and not ("Red Fox Genome Sheds Light on Domesticated Dogs (and Maybe Humans)"; "Lowland Gorilla Genome Sequenced"; "Giraffe Genome Reveals Relevant Adaptations"; "Genome Structure of the American Cockroach"). Recently, a group of researchers from the U.S., Canada, Europe, and the United Kingdom published the results of their studies with the blue whale, Balaenoptera musculus, which while being "the largest animal to have ever existed, reaching up to 110 feet in length and weighing 330,000 pounds" does not have the largest eukaryotic genome (at 2.7 billion base pairs (Gbps), compared with Paris japonica at 149 Gbps and the Australian lungfish at 43 Gbps); see Bukhman et al., 2024, A high-quality blue whale genome, 1 segmental duplications, and historical demography, Molec. Biol. Evol. Vol. 41, No. 3; https://doi.org/10.1093/molbev/msae036).
The paper acknowledges the existence of four subspecies of blue whales that are currently recognized: three found in the Southern Hemisphere and northern Indian Ocean, and the fourth (an individual from which produced the "high-quality" genomic information they disclose*) that includes blue whales in the North Atlantic and North Pacific. The results presented here suggest that these two populations began to diverge 100–200,000 years ago, and became "completely genetically isolated" from one another at the time of the last interglacial period (about 20,000 years ago).
One of the goals of the research is related to the observation that large animals seem to have developed mechanisms to resist cancer (a phenomenon termed Peto's Paradox). Previous genome sequencing of large animals had revealed mutations and duplications of tumor suppressor genes and genes involved in DNA repair and apoptosis, which may account for the observed biology (large mammals, which by having more cells that would suggest higher susceptibility to cancer). Previous studies had also identified segmental duplications (SDs) associated with longevity and increased body size in cetaceans and elephants and duplicated genes and gene families in cetacean genomes using short-read and long-read sequencing methods.
Here, the assembled blue whale genomic sequences obtained by these researchers could be assigned to 23 chromosomal-level scaffolds from the 21 whale autosomes plus the X and Y chromosomes (the sample was a male, although sequencing of the Y chromosome was incomplete). Comparative analysis was performed between the blue whale genome and the vaquita (Phocoena sinus), bottlenose dolphin (Tursiops trunca), and cattle (Bos taurus) genomes which are evolutionarily related as members of the placental mammal Order Artiodactyla.
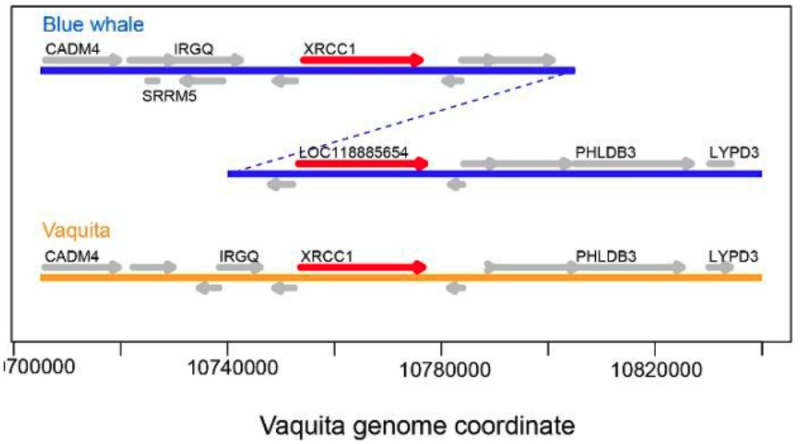
The assembly showed both a high level of completeness and revealed gene duplications and expansions in the blue whale genome. This research revealed an evolutionarily recent burst in segmented duplications (SDs) that were correlated with body size in cetaceans. SDs detected in the blue whale genome are "gene rich, amounting to a roughly 7.1-fold burst in gene duplications relative to vaquita and dolphin, and 3.0-fold relative to cattle." These researchers detected 234 duplicated genes in the blue whale, 167 in the vaquita, 211 in dolphin, and 205 in cattle. The distribution of amplified genes showed that "the ten most highly amplified genes account[ed] for 331 gene copies out of 700 (47%) total duplicated genes." The blue whale sample showed 46 genes having more than 4 copies, compared with 9 in dolphin, 8 in cattle, and 6 in vaquita. It had been shown in previous studies that in cattle there are 163 loci associated with body size, and these researchers found 52 corresponding loci in blue whales and 53 such loci vaquita. In particular comparisons between blue whale and vaquita were identified 133 duplicated genes of potential interest that included KCNMB1, which contained ancient (>20 Mya) duplication events. Other genes detected as duplications in the whale genome include ones related to longevity (MT1X), body size (CHRNB1, DPEP2), development (FZD5, CDK20), cancer (C2orf78, FZD5, DDX24, NCAM1, MT1X, XRCC1, CDK20), obesity and diabetes (DPEP2), and the immune system (NCAM1), with certain of them having been greatly amplified (such as XRCC1, CDK20, and CHRNB1x). A comparison between the genomic regions encoding XRCC1 in blue whale was shown in this figure, illustrating genomic rearrangement in whale not found in vaquita:
A specific gene found to be amplified in blue whale was Insulin-like growth actor 1 (IGF-1), which has been associated with large body size in dogs (see "From Toy Poodle to Rottweiler: Why Is Fido So Small (or Large)?"). Single nucleotide polymorphism (SNP) analysis was performed on a specific SNP associated with size variation and showed a lack of the variant responsible for large (CA) and small (CG) size, sharing with humans, artiodactyls and other mammals the same sequence (AG) in this SNP. Accordingly, multiple alignment of IGF-1 sequences were performed on 11 cetacean and 18 land artiodactyl species, wherein the 11 cetaceans fell into three phylogenetic clades illustrated by this phylogenetic tree:

wherein the first clade comprises large baleen whales, blue (Balaenoptera musculus) and minke (B. acutorostrata); second, a giant toothed whale, the sperm whale Physeter catodon; and third, smaller toothed whales, including orca (Orcinus orca), dolphins, and porpoises. These studies revealed that three large whales (blue, minke, and sperm), have a different allele from all other artiodactyls found at two sites in the intergenic region upstream of the IGF-1 gene, four in the second intron, and one in the third or fourth intron, depending on the IGF1 isoform. The orca was found to have the same allele as its smaller relatives at these sites. Also found where sites where the four largest whales (blue, minke, sperm) and orca, have a different nucleotide compared to all smaller cetaceans, with there being four such sites in the second intron and one in the third intron. The authors suggest that the large whales have the ancestral allele, while the small ones have evolved an alternative and note that the orca, while being more closely related to smaller dolphins, has the allele characteristic of baleen and sperm whales.
The paper also discusses their "historical demography analysis that suggested a population division between Pacific and Atlantic blue whales." These population metrics were substantially the same between the Pacific and Atlantic populations of blue whale until the end of the Saalian ice age about 125 million years ago. The Atlantic population then showed a slight increase over the Pacific population until the last glacial maximum (LGM) about 20,000 years ago, according to the paper, when both populations decreased. Pacific and Atlantic blue whale populations were found to be highly heterozygous and genetically isolated since the last interglacial period, with blue whales in the North Atlantic and eastern North Pacific beginning to diverge around 100-200,000 years ago.
"Runs of homozygosity analysis" revealed that since that time there has been a low level of inbreeding in the blue whale population, the paper explaining that "[r]uns of homozygosity (ROH) are indicative for the frequency and relative timing of inbreeding events and were frequently used to assess the impact of inbreeding on different mammalian and cetacean populations." The data obtained showed 108 runs that were 500 kb or longer in both Pacific and Atlantic populations, and that over 70% of these ROH were shorter than 1 Mb, which indicated that "most ROH were probably fragmented over time by frequent outcrossing." According to the paper, the "high heterozygosity and short runs of homozygosity in the blue whale genome suggest a large and outbred population in the Northern Pacific."
The paper concludes by asserting that "[a] major finding of this work is the presence of large copy number expansions of a number of genes in the blue whale, while relatively few such expansions are observed in vaquita, bottlenose dolphin, and cattle [which] expansions [in whales] are recent in evolutionary time" and "the assembly will serve as a valuable resource to the scientific community, enabling future comparative genomics studies to further our understanding of large animal longevity and associated resistance to cancer, and helping conserve this magnificent species."
For those interested in methodology used by these researchers the paper discloses the following:
* Our assembly has been annotated by NCBI. Additionally, we annotated both primary and alternate pseudohaplotype by projecting human and mouse genes using TOGA (Tool to infer Orthologs from Genome Alignments). We also predicted GO terms for all protein coding genes identified by the NCBI Eukaryotic Genome Annotation Pipeline using Phylo-PFP.

Leave a comment