By Kevin E. Noonan –
 "This application claims priority to [properly identified earlier-filed application, the disclosure of which is expressly incorporated herein in its entirety" is a phrase commonly found in patents and patent applications as an attempt to bolster disclosure without burdening the specification unnecessarily. Like many (most) stratagems, use of this phrase can give rise to unexpected (and unwanted) implications, as was noted in a Federal Circuit opinion affirming a decision by the Patent Trial and Appeal Board in inter partes review proceedings instituted at the behest of challenger ModernaTX that invalidated all claims of the challenged patent owned by Arbutus in Arbutus Biopharma Corp. v. ModernaTx, Inc.
"This application claims priority to [properly identified earlier-filed application, the disclosure of which is expressly incorporated herein in its entirety" is a phrase commonly found in patents and patent applications as an attempt to bolster disclosure without burdening the specification unnecessarily. Like many (most) stratagems, use of this phrase can give rise to unexpected (and unwanted) implications, as was noted in a Federal Circuit opinion affirming a decision by the Patent Trial and Appeal Board in inter partes review proceedings instituted at the behest of challenger ModernaTX that invalidated all claims of the challenged patent owned by Arbutus in Arbutus Biopharma Corp. v. ModernaTx, Inc.
The subject matter of the challenged patent, No. 9,404,127, was stable nucleic acid lipid particles ("SNALP") that occur in two morphologies: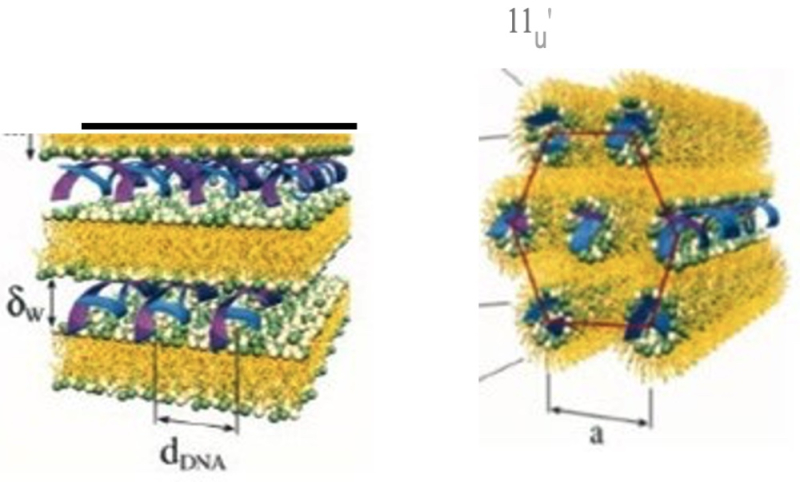
wherein the structure illustrated on the left is "lamellar" and the structure illustrated on the right is "non-lamellar"; the '127 patent claims are directed to the non-lamellar form. This "morphology limitation" is dependent upon how the SNALP is made, which is in turn dependent upon the lipids used in making the SNALP and the process used. With regard to the process SNALPS can be made by a Stepwise Dilution Method (SDM) or a Direct Dilution Method (DDM) and the formulations can have five different ratios for the composite lipids, with 1:62 and 1:57 being those relevant to the claims at issue and referring to a conjugated lipid and a cationic lipid, respectively. Independent claim 1 was reproduced in the opinion as being representative:
1. A composition comprising:
a plurality of nucleic acid-lipid particles, wherein each particle in the plurality of particles comprises:
(a) a nucleic acid;
(b) a cationic lipid;
(c) a non-cationic lipid; and
(d) a conjugated lipid that inhibits aggregation of particles, wherein at least about 95% of the particles in the plurality of particles have a non-lamellar morphology (with this limitation being the morphology limitation).
The '127 patent issued from an application filed on March 9, 2015 that claimed priority to a provisional application filed on June 30, 2010 (making June 30, 2009 the critical date for novelty-destroying prior art). The '127 patent incorporated by reference published U.S. Patent Application Publication Nos. 2007/0042031 ("the '031 application, for disclosure of DDM) and 2004/0142025 (for SDM and apparatuses therefor), these publications being indisputably in the prior art at the '127 patent's earliest claimed priority date.
The PTAB held all claims of the challenged '127 patent to be invalid as being anticipated by U.S. Patent No. 8,058,069 ("the '069 patent"), which was filed on April 15, 2009 and claimed priority to a provisional application filed one year earlier (the Federal Circuit noting that the '127 patent did not claim priority to the '069 patent, even though these patents are commonly owned by Arbutus). The basis for this decision was that the Board found that both patents:
[A]re directed to the same purpose (providing SNALP, methods of making and delivering SNALP); disclose at least the 1:57 and 1:62 formulations; explain that SNALP can be formed by any method in the art including direct dilution, and direct the reader to rely on the '031 publication for details on using DDM.
The '069 patent incorporated by reference several other patents that the Board held disclosed "several of the same disclosures and experiments" set forth in the '127 patent.
The Board's decision focused on the claim 1(d) element (the morphology limitation) and whether it was inherently disclosed in the '069 patent. The basis for Moderna's assertion of inherent anticipation was that the non-lamellar structure arose as a consequence of the composition of the SNALP and the method (DDM) used to produce it which the Board found convincing (despite expert testimony based on experimental evidence to the contrary), and Arbutus's concession that the specification of a continuation of the '069 patent disclosed the morphology limitation. Accordingly, the Board held all claims of the '127 patent to be invalid for anticipation. This appeal followed.
The Federal Circuit affirmed in an opinion by Judge Reyna, joined by Judges Schall and Chen. With regard to the question of incorporation by reference, the panel cited its precedent that "[w]hen a reference or material from various documents is incorporated, they are 'effectively part of the host document as if [they] were explicitly contained therein,'" Advanced Display Sys., Inc. v. Kent State Univ., 212 F.3d 1272, 1282 (Fed. Cir. 2000). The panel, like the Board, rejected Arbutus's arguments that the DDM process comprised "many parameters that could be varied" based on Arbutus's expert's concession that the '435 patent (a continuation of the '069 patent) also disclosed the morphology limitation. In the circumstances before the Court, "the disclosure of the '069 patent and its incorporated references sufficiently demonstrate to a person skilled in the art how to make and use the claimed compositions, processed by DDM, that results in the Morphology Limitation." The evidence before the Board satisfied the legal requirement for inherent anticipation that production of the claimed SNALPs was a "natural result flowing from" the disclosure in the prior art under SmithKline Beecham Corp. v. Apotex Corp., 403 F.3d 1331, 1343–44 (Fed. Cir. 2005). Here, the panel asserts that "the '127 and '069 patents disclose the same formulations with 'almost identical wording,'" ("[t]he specificity of the disclosure in the '069 patent is the same as in the '127 patent") including the 1:57 and 1:62 formulation ratios (and that the other ratios disclosed and claimed in the '127 specification could be substituted without impacting the morphology limitation according to Arbutus's expert. Both the '127 and '069 patents reference the '031 application for disclosure of the DDM method for producing SNALPs having the claimed morphology limitation with the challenged '127 patent incorporating by reference the '031 disclosure, which supported the Board's conclusion that the '127 patent discloses this method the same way it was disclosed in the prior art '031 application. The opinion concludes that because the panel found no error in the factual question of whether the prior art taught "the same formulations and the same DDM" disclosed and claimed in the '127 patent the challenged claims were anticipated by the art and invalid.
With regard to dependent claims directed to forms of the claimed SNALPs comprising mRNA (claim 3) or "fully encapsulated" nucleic acids (claim 8) were disclosed as formulations in the prior art. The Court found SNALPs having specific three-dimensional structures (claim 9) to recite inherent properties of the SNALPs produced according to the cited art. And regarding claims to percent ranges for the lipid components of the claimed SNALPs (claims 10-12), the Court relied on its precedent that "[w]hen a patent claims a chemical composition in terms of ranges and a single prior art reference discloses a composition that falls within each of the ranges, the range is anticipated, citing Titanium Metals Corp. of Am. v. Banner, 778 F.2d 775, 782 (Fed. Cir. 1985). This disclosure arose in the cited prior art by incorporation by reference of U.S. Patent Application Publication No. 2006/0083780 (the "'780 publication"), U.S. Patent Application Publication No. 2004/0142025 (the "'025 publication"), and U.S. Patent No. 5,885,613 (the "'613 patent").
While not dispositive, this decision is relevant to the on-going disputes between owners of lipid nanoparticle IP and vaccine makers over COVID 19 and other mRNA-based vaccines against other diseases (see "Pfizer and BioNTech Sued for Patent Infringement over mRNA Vaccine").
Arbutus Biopharma Corp. v. ModernaTx, Inc. (Fed. Cir. 2023)
Panel: Circuit Judges Reyna, Schall, and Chen
Opinion by Circuit Judge Reyna

Leave a comment