By Kevin E. Noonan —
The parties in Interference No. 106,132, namely Senior Party Sigma-Aldrich and Junior Party the University of California/Berkeley, the University of Vienna, and Emmanuelle Charpentier (collectively, "CVC"), filed their respective lists of proposed preliminary motions four days prior to their August 3rd teleconference with the Board to present their arguments for the Board to grant leave to file any of them.
Junior Party CVC proposed ten preliminary motions. CVC Substantive Preliminary Motion No. 1, which CVC characterized as a "threshold" motion, sought to have a finding of no interference-in-fact because CVC alleged Sigma-Aldrich's application-in-interference was properly governed under the "first inventor to file" provisions of the Leahy-Smith America Invents Act because that patent "contains or contains at any time" (emphasis in brief) subject matter not entitled to priority to an application filed prior to enactment of the AIA. As Sigma-Aldrich did in its analogous request for preliminary motion against Broad (see "Sigma-Aldrich and Broad Propose Preliminary Motions in Recent CRISPR Interference No. 106,133"), CVC provides an Appendix outlining the bases in Sigma-Aldrich's application-in-interference No. 15/456,204:
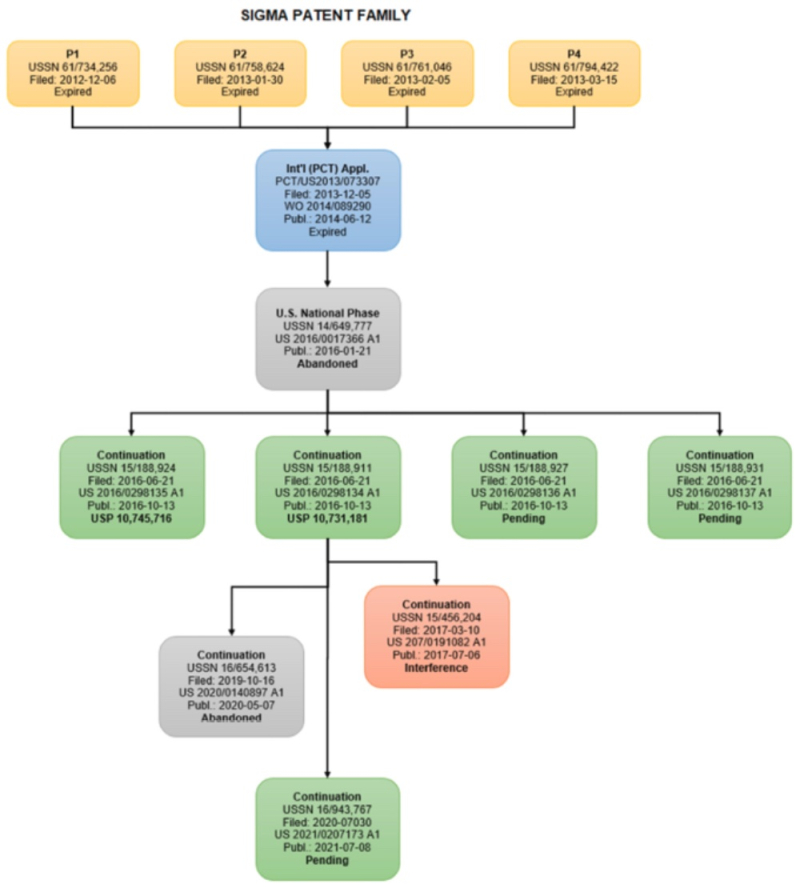
CVC also provides as an example in the text amendment of a claim during prosecution of a parent application (No. 15/188,911) that the Office determined was sufficient for the amended claim not to be entitled to the first-to-invent provisions of the 1952 Patent Act. This determination regarding the '911 application, from which the '204 application claimed priority, was enough for the Board to conclude, according to CVC, that the '204 application was not entitled to participate in this interference. In addition to asking the Board for leave to file its Motion, CVC asks that this motion be authorized, briefed, and decided before any other motions are briefed or considered, so as not to burden the parties in filing unnecessary motions.
CVC's Substantive Preliminary Motion No. 2 under 37 C.F.R. §§ 41.121(a)(1)(ii) and 41.208(a)(3) asks leave to file a motion for benefit of priority to U.S. provisional application Nos. 61/652,086, filed May 25, 2012 ("P1"); 61/716,256, filed October 19, 2012 ("P2"), earlier-filed applications from which CVC has sought priority benefit in Interference Nos. 106,115 (where the Board denied the motion) and 106,127 (where the Board has not yet ruled). CVC asks for a longer page limit for its motion, "[d]ue to the complexity of the issues and extensive evidentiary record implicated by this Motion." CVC also argued that the Board's adverse decision on this motion in the '115 does not raise an estoppel because that decision is not yet final (having not been ripe for appeal) and because the Count in the '115 Interference and the Count in this interference are not identical.
CVC's Substantive Preliminary Motion No. 3 seeks to have the Board deny Sigma-Aldrich benefit of priority to its earliest provisional application, No. 61/734,256 filed December 6, 2012 (the Board having given Sigma-Aldrich the priority benefit to this application in the Declaration.
CVC's Substantive Preliminary Motion No. 4 asks the Board to change the Count, "to better—and more uniformly—reflect the common invention being adjudicated in multiple pending interferences involving the claims of multiple parties directed to sgRNA CRISPR-Cas9." CVC notes in particular that Sigma-Aldrich's "half" of the Count does not contain the limitation in claim 33 of the '204 application "wherein the guide RNA is a single molecule," while CVC's half of the Count is expressly limited to eukaryotic CRISPR species encompassing single molecule RNA species. CVC relies on 37 C.F.R. § 41.1(b) for "considerations of efficiency, uniformity, and clarity [to] justify narrowing Sigma's half of the Count."
CVC then proposes three separate Substantive Preliminary Motions under 37 C.F.R. § 41.121(a)(1) for unpatentability of Sigma-Aldrich's claims in this interference. CVC's Substantive Preliminary Motion No. 5 asks the Board 37 C.F.R. § 41.121(a)(1) to find the claims of the '204 application to be unpatentable under AIA 35 U.S.C. § 103 for obviousness over U.S. Application Publication No. 2015/0322457, alone or in combination with several prior art references "disclosing gene editing using donor integration-based strategies"; CVC relies on its Substantive Preliminary Motion No. 1 that the '204 application is not entitled to the benefits of the first to invent provisions of the 1952 Patent Act in support of this motion. In addition, CVC argues a variety of public and PTAB policy goals purportedly furthered by the Board granting this motion.
CVC's Substantive Preliminary Motion No. 6 asks the Board 37 C.F.R. § 41.121(a)(1) to find the claims of the '204 application to be unpatentable under AIA 35 U.S.C. § 102 or § 103 for anticipation or obviousness over U.S. Application Publication No. 2014/0068797, alone or in combination with several prior art references in view of Sigma-Aldrich's purported inability to swear behind these references based on CVC's arguments in its Substantive Preliminary Motion No. 1. CVC particularly notes that the basis for the Office finding the claims in the '204 application patentable over cited prior art was that the claims were amended to recite CRISPR embodiments having the feature of integrating donor DNA into target DNA. This feature is found, CVC argues, in its '797 Publication as well as its P1 and P2 provisional applications, none of which were considered during ex parte prosecution. CVC also argues that any adverse decision in the '115 Interference does not estop their making this argument in this interference, inter alia, because that earlier decision is not final.
CVC's Substantive Preliminary Motion No. 7 asks the Board 37 C.F.R. § 41.121(a)(1) to find the claims of the '204 application to be unpatentable under AIA 35 U.S.C. § 102 or § 103 for anticipation or obviousness over under a combination of prior art references including "Jinek 2013, Mali 2013, Hwang 2013, Cong 2013, and/or Cho 2013." In addition to asserting invalidity, CVC asserts that Sigma-Aldrich cannot "swear behind" these references due to its status as not being entitled to the first to invent provisions of the 1952 Patent Act.
CVC asks the Board's leave to file two miscellaneous motions. The first, CVC's Contingent Miscellaneous Motion No. 8 under 37 C.F.R. § 41.121(a)(3), asks the Board to include to add claims of U.S. Patent Nos. 10,731,181 and 10,745,716, contingent on the Board either denying it authorization to CVC for filing its Preliminary Motion No. 1 or denying the motion on its merits.
CVC's Contingent Miscellaneous Motion No. 9 under 37 C.F.R. § 41.121(a)(3) asks the Board to add claims in Sigma-Aldrich's U.S. Patent Application Nos. 15/188,927; 15/188,931; 16/943,767; and 17/208,477 to this Interference. Like CVC Motion No. 8, this motion is contingent on the Board either denying it authorization to CVC for filing its Preliminary Motion No. 1 or denying the motion on its merits.
Finally, CVC's Substantive Preliminary Motion No. 10 was a motion under 37 C.F.R. § 41.208(a)(4) seeking judgment based on priority.
Sigma-Aldrich filed its request under 37 C.F.R. § 41.121(a)(1)(i) to change the Count to one of five different alternatives. Sigma-Aldrich justifies this request by asserting that "[t]he current Count 1, as set forth in the Declaration, encompasses two patentably distinct inventions: (1) CRISPR-Cas9 in a eukaryotic cell to cleave a target DNA; and (2) CRISPR-Cas9 in a eukaryotic cell to cleave a target DNA and subsequently to integrate a donor DNA sequence into the target DNA." But the step of "subsequently to integrate a donor DNA sequence into the target DNA" is not obvious over merely CRISPR-mediate cleavage of target DNA and thus this aspect is patentably distinct. Citing 37 C.F.R. § 41.201 for the principle that a single interference Count "should not encompass two patentably distinct inventions," Sigma-Aldrich argued that CVC's part of the Count is limited to CRISPR-mediated target DNA cleavage alone while its own portion of the Count is limited to CRISPR-mediated integration of donor DNA into the target site. The inequity raised by this Count structure is that it would enable CVC to submit proofs of its invention (limited to DNA cleavage) to prevail on separately patentable subject matter encompassing donor DNA integration.
Sigma-Aldrich's Proposed Counts 2-5 are as follows:
Proposed Count 2 (all proposed Counts recite claim 31 of Sigma-Aldrich's Application No. 15/456,204 as in the Count as declared):
156. A method of cleaving or editing a target DNA molecule or modulating transcription of at least one gene encoded thereon, the method comprising:
contacting a target DNA molecule having a target sequence with an engineered and/or non-naturally-occurring Type II Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)—CRISPR associated (Cas) (CRISPR-Cas) system comprising:
a) a single molecule DNA-targeting RNA comprising
i) a targeter-RNA that hybridizes with the target sequence, and
ii) an activator-RNA that hybridizes with the targeter-RNA to form a double-stranded RNA duplex of a protein-binding segment,
wherein the targeter-RNA and the activator-RNA are covalently linked to one another with intervening nucleotides; and
b) a Cas9 protein,
wherein the single molecule DNA-targeting RNA forms a complex with the Cas9 protein, thereby targeting the Cas9 protein to the target DNA molecule
whereby said target DNA molecule is cleaved or edited or transcription of at least one gene encoded by the target DNA molecule is modulated, and
wherein said contacting occurs in a eukaryotic cell.
157. The method of Claim 156, wherein, prior to the contacting step, the method comprises:
introducing into the eukaryotic cell containing the target DNA molecule:
1) the single molecule DNA-targeting RNA, or a DNA molecule comprising a nucleotide sequence that (i) encodes the single molecule DNA targeting RNA and (ii) is operably linked to a regulatory element operable in said eukaryotic cell; and
2) the Cas9 protein, an RNA molecule comprising a nucleotide sequence encoding the Cas9 protein, or a DNA molecule comprising a nucleotide sequence that (i) encodes the Cas9 protein and (ii) is operably linked to a regulatory element operable in said eukaryotic cell.
164. The method of Claim 157, wherein the method comprises creation of a double strand break in the target DNA molecule which is repaired by a homology-directed repair mechanism which incorporates a sequence of a donor polynucleotide into the target DNA molecule, thereby editing the target DNA molecule.
(CVC Application No. 15/947,680)
Sigma-Aldrich notes that an advantage of this Proposed Count 2 is that it reconciles the subject matter categories of the two alternatives in the Count (both are directed to methods) instead of Count 1 as declared (directed to CVC's composition and Sigma-Aldrich's method).
Sigma-Aldrich's Proposed Count 3 is the same as Proposed Count 2 but Claim 164 would depend directly on claim 156 instead of including the limitations of intervening claim 157 of CVC's '680 application).
Sigma-Aldrich's Proposed Count 4 recites CVC's claim 169 (dependent on claim 156) in the alternative to Sigma-Aldrich's claim 31 of the '204 application:
169. The eukaryotic cell of Claim 156, wherein the system comprises a donor polynucleotide and the system is capable of editing the target DNA molecule by inserting a sequence of the donor polynucleotide into a cleaved strand of the target DNA molecule.
Sigma-Aldrich's Proposed Count 5 recites in alternative composition of matter claims; this proposal constitutes CVC's claim 169 of its Application No. 15/981,807 and Sigma-Aldrich's claim 64 of the '204 application:
64. A eukaryotic cell comprising a chromosomal sequence and a Clustered Regularly Interspersed Short Palindromic Repeats (CRISPR)/CRISPR associated (Cas) (CRISPR-Cas) type II system comprising
(i) a CRISPR-Cas type II protein linked to only one nuclear localization signal (NLS) or a nucleic acid encoding the CRISPR-Cas type II protein linked to only one NLS, wherein the CRISPR-Cas type II protein is a Cas9 protein, and the nucleic acid encoding the CRISPR-Cas type II protein is codon optimized for expression in the eukaryotic cell;
(ii) a guide RNA or DNA encoding the guide RNA, wherein the guide RNA comprises a first region that is complementary to a target site in the chromosomal sequence, which target site in the chromosomal sequence is immediately followed by a protospacer adjacent motif (PAM), and a second region that interacts with the CRISPR-Cas type II protein, and wherein the guide RNA comprises a crRNA and a tracrRNA; and
(iii) a donor polynucleotide comprising a donor sequence and upstream and downstream sequences;
wherein the guide RNA is capable of guiding the CRISPR-Cas type II protein to the target site in the chromosomal sequence, the CRISPR-Cas type II protein is capable of introducing a double-stranded break at the target site, and the system is capable of repairing the double-stranded break by a DNA homology-directed repair (HDR) process leading to integration or exchange of the donor sequence into the chromosomal sequence.
Sigma-Aldrich requested leave to file its Contingent Motion No. 2 to deny CVC benefit of Application 61/757,640 (filed Jan. 28, 2013) ("CVC P3") or 61/765,576 (filed Feb. 15, 2013) ("CVC P4") under 37 C.F.R. § 41.121(a)(1), should the Board grant any of alternative Proposed Counts 2, 3, 4, or 5, on the grounds that these applications do not provide a constructive reduction to practice of an invention recited by any of these alternative Counts, i.e., "use of a CRISPR-Cas9 composition or method in a eukaryotic cell to successfully cleave a target DNA and thereafter to integrate a donor DNA sequence into that cleaved target DNA."
Sigma-Aldrich also sought leave to file its Substantive Preliminary Motion No. 3 for a Board determination that CVC's involved claims are unpatentable under §§ 102(a)/(e) and/or 103 in view of the Chen reference, WO 2014/087489290 A1 (published June 12, 2014), which claimed priority to Application 61/734,256 (filed Dec. 6, 2012) ("Sigma P1").
Sigma-Aldrich also seeks a Protective Order such as the one entered in other CRISPR interferences, and finally filed a motion under 37 C.F.R. § 41.208(a)(4) seeking judgment based on priority.
In addition, Sigma-Aldrich adds the Board to address some "miscellaneous administrative issues" such as deadlines for filing and service in this interference and asks the Board to waive the requirement for a separate Statement of Material Fact or not have these pages be included in the page limit, as well as a few typographical errors in the caption.
Future posts will discuss the parties arguments at the August 3rd teleconference and the Board's determinations regarding which Preliminary Motions the parties will be permitted to file.

Leave a comment