By Andrew Williams —
In addition to the various associations, academics, and interest groups that filed "supplemental" amici curiae briefs in Association for Molecular Pathology v. U.S. Patent and Trademark Office ("the Myriad case"), two briefs were filed by companies that would be directly negatively impacted if either "the isolated DNA claims [or the] method claim 20 of the '282 patent" were found to be patent ineligible. This is because, as one of the briefs put it, a broad application of patent ineligibility "could disrupt the expectations of large numbers of chemical and biotechnology patent holders and researchers who have depended on the patent system to secure rights to valuable intellectual property and to attract necessary capital and investment." Therefore, these organizations have "a strong interest in ensuring the stability of the patent system as it relates to chemical and biotechnology inventions."
 The above language comes from an amici brief filed by research-based biopharmaceutical companies Gilead Sciences, Inc., Confluence Life Sciences, Inc. and Euclises Pharmaceuticals, Inc. (collectively "Gilead"). Gilead's brief is directed solely to the issue of "whether a synthetic, man-made sequence of nucleotides forming a 'composition of matter,' which is both 'new and useful," is § 101-includable," and therefore it focuses exclusively on the cDNA sequence defined in claim 2 and recited as SEQ ID NO:1 of U.S. Patent No. 5,747,282 ("the '282 patent"). However, Gilead is quick to point out that this is not a "gene patenting" case — most likely because the synthetic sequence of nucleotides invented by Myriad Genetics, Inc. ("Myriad") never existed in nature prior to its invention. Gilead alleges that the lower court (the District Court of the Southern District of New York) erred as a matter of law for failing to recognize the "made-by-man" standard for determining whether a new chemical substance satisfies 35 U.S.C. § 101, and erred as a matter of fact by misapplying the "markedly different" test. Gilead concludes by arguing that nothing in the Supreme Court's Prometheus decision changes the Federal Circuit's July 2011 decision that claim 2 of the '282 patent is § 101-eligible.
The above language comes from an amici brief filed by research-based biopharmaceutical companies Gilead Sciences, Inc., Confluence Life Sciences, Inc. and Euclises Pharmaceuticals, Inc. (collectively "Gilead"). Gilead's brief is directed solely to the issue of "whether a synthetic, man-made sequence of nucleotides forming a 'composition of matter,' which is both 'new and useful," is § 101-includable," and therefore it focuses exclusively on the cDNA sequence defined in claim 2 and recited as SEQ ID NO:1 of U.S. Patent No. 5,747,282 ("the '282 patent"). However, Gilead is quick to point out that this is not a "gene patenting" case — most likely because the synthetic sequence of nucleotides invented by Myriad Genetics, Inc. ("Myriad") never existed in nature prior to its invention. Gilead alleges that the lower court (the District Court of the Southern District of New York) erred as a matter of law for failing to recognize the "made-by-man" standard for determining whether a new chemical substance satisfies 35 U.S.C. § 101, and erred as a matter of fact by misapplying the "markedly different" test. Gilead concludes by arguing that nothing in the Supreme Court's Prometheus decision changes the Federal Circuit's July 2011 decision that claim 2 of the '282 patent is § 101-eligible.
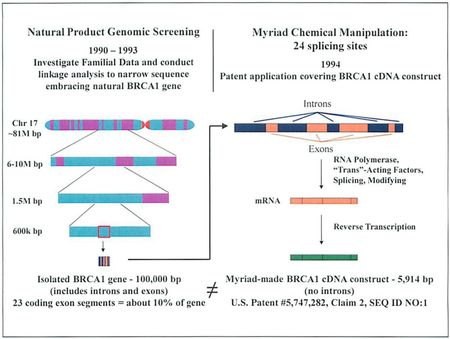
Gilead begins by pointing out all of the transformative steps that Myriad had to undergo in order to make SEQ ID NO:1. First, Myriad researchers identified that genetic defects in the BRCA1 gene were the likely source of a pre-disposition to breast and ovarian cancer. Further, before any chemical transformative step was undertaken, the researchers used natural product genomic screening to narrow Chromosome 17 (with about 81 million base pairs) to a 100,000 base pair isolated BRCA1 gene. This gene, of course, includes both introns and exons (with 23 coding exon segments). Therefore, Myriad had to chemically manipulate, or transform, the isolated gene to derive its cDNA construct (as shown in the below figure from Gilead's brief).
Gilead alleges legal error on the part of the lower court because, as the Supreme Court reiterated in Diamond v. Chakrabarty, "Congress intended statutory subject matter to 'include anything under the sun that is made by man.'" In fact, Gilead provides a reminder of the broad statutory goal of the patent system, pointing out that it was Jefferson's philosophy that "ingenuity should receive a liberal encouragement." Therefore, even though patent eligibility has clear limits, the fundamental question "should be whether the invention is truly 'made by man," i.e., whether the invention is 'the result of human ingenuity and research.'" Gilead supports this proposition by citing to eleven Federal Circuit, CCPA, and District Court cases that found synthetic compounds and materials patent eligible, and six Federal Appellate and District Court cases that found purified compounds to be patent eligible.
In contrast, the Gilead brief does not spend much effort explaining why the lower court erred as a matter of fact by misapplying the "markedly different" test. Instead, it simply points out that, to the extent the Chakrabarty decision imposed a "markedly different characteristics" requirement into § 101, the Federal Circuit had already found that this requirement was satisfied in its July 2011 decision. The brief also reiterates the practical utility of new DNA molecules at least as a diagnostic tool, which is evidenced by Myriad's medical and economic success using the synthetic cDNA compound as a predictor of genetic pre-disposition to cancer.
Gilead's conclusion, however, focuses exclusively on explaining why the Prometheus decision does not apply to Myriad's synthetic cDNA. First, unlike Prometheus's method claims, there were at least 20 transformative steps that resulted in a sequence of nucleotides that had not, and could not, exist in nature. Second, these transformative steps fully meet the Diehr standard of not being mere applications of "mathematical formula" or "laws of nature."
 Protein Sciences Corporation ("PSC), another biopharmaceutical company, filed a separate amicus brief. However, instead of limiting its analysis to the DNA claims, PSC addresses all of the claims that were originally at issue. Importantly, PSC explains how the "Growth Rate Claim" continues to be patent eligible, even after the Prometheus decision.
Protein Sciences Corporation ("PSC), another biopharmaceutical company, filed a separate amicus brief. However, instead of limiting its analysis to the DNA claims, PSC addresses all of the claims that were originally at issue. Importantly, PSC explains how the "Growth Rate Claim" continues to be patent eligible, even after the Prometheus decision.
According to PSC, Prometheus reduced the patent-eligibility question to the following test:
(1) Is the claim a method or process claim? If yes, then:
(2) Does the method or process call for applying a law of nature? If yes, then:
(3) Do the steps of the method or process:
a. Merely call for a particular audience to apply the law of nature or for applying the law of nature in a particular technological environment, or
b. Call for "[p]urely 'conventional or obvious' '[pre]-solution activity'"?
If the answer to either (3)(a) or (3)(b) is "yes," then the method or process fails to be patent eligible, and also fails to meet § 112, ¶ 1. If either question cannot be answered because of an insufficient record as to whether the steps are "conventional or obvious," the case should be remanded to develop the record. This is, of course, a very narrow reading of Prometheus (suggesting that any composition of matter would be patent eligible), but PSC's application of Prometheus to the present claims is rooted in this understanding. PSC also finds it necessary to define "law of nature," which according to the 1993 New Shorter Oxford English Dictionary is a "scientific generalization based on empirical observation" (as paraphrased by PSC).
Therefore, the analysis as to whether isolated DNA or cDNA are patent eligible according to Prometheus is simple — they are compositions of matter, and therefore Prometheus does not apply. However, apparently to be safe, PSC also explains that "isolated DNA molecules are chemical compositions that possess physical, chemical and structural properties that differ from their naturally-occurring counterparts, and are molecules that man must create." In addition, PSC points out that isolated DNA has new properties not shared by its native counterpart, including uses as a probe, a diagnostic tool, a primer, and in sequencing. Thus, as to part 2 of the alleged Prometheus test, they are not products of nature. To further bolster its conclusion, PSC points out that this conclusion comports with the longstanding practice of the PTO and the Federal Circuit. PSC's analysis of cDNA is similar to that of isolated DNA, and the same conclusion is reached — cDNA is patent eligible.
As for the method claims, PSC first notes that Myriad's "analyzing" and "comparing" claims, which the Federal Circuit previous held to be not patent eligible, would also fail the Prometheus test. However, the Growth Rate Claim, claim 20 of the '282 patent, would be patent eligible under this test. The claim at issue reads:
20. A method for screening potential cancer therapeutics which comprises: growing a transformed eukaryotic host cell containing an altered BRCA1 gene causing cancer in the presence of a compound suspected of being a cancer therapeutic, growing said transformed eukaryotic host cell in the absence of said compound, determining the rate of growth of said host cell in the presence of said compound and the rate of growth of said host cell in the absence of said compound and comparing the growth rate of said host cells, wherein a slower rate of growth of said host cell in the presence of said compound is indicative of a cancer therapeutic.
Because this claim is a method claim, question (1) of the test is answered in the affirmative. However, according to PSC, the method uses transformed cells that contain an altered BRCA1 gene, which are not naturally occurring (after all, even the claim language itself indicates a "transformation" has occurred). Moreover, because these cells do not occur naturally, they cannot be calling for the application of a law of nature. Therefore, PSC concludes that this claim is more akin to the claim in Diehr (applying the Arrhenius equation) than the claims in Prometheus.
It is unclear if either of these briefs adds anything new to the discussion. However, it is clear that each of these four companies, and many others like them, have invested substantial research and development (and therefore money and time) with the understanding that the patent system will protect their investments. If the Federal Circuit were to take a narrow view of patent eligibility after the Prometheus case, it could seriously negatively impact the development and investment of future biopharmaceuticals and vaccines. As such, hopefully the Federal Circuit will at least consider the perspective of these companies.
For additional information regarding this topic, please see:
• "Health Care Professionals Contend That Isolated DNA and cDNA Are Patent Ineligible," July 16, 2012
• "Coalition of Amici File Brief in Support of Myriad," July 15, 2012
• "Dr. James Watson: Human Genes Should Not Be Patented," July 12, 2012
• "Scientist-Law Professor Files Amicus Brief in Myriad Case," July 11, 2012
• "U.S. Government: Mayo Decision Supports Prior Argument That Isolated Genomic DNA Is Not Patent Eligible," July 10, 2012
• "IPO Amicus Brief Argues for Patent Eligibility of Myriad's Isolated DNA Claims and Method Claim 20," July 9, 2012
• "Eli Lilly & Co. File Amicus Brief in AMP v. Myriad," June 27, 2012
• "Parties and Amici File Briefs in Myriad Case," June 17, 2012

Leave a comment