By Kevin E. Noonan —
The future is always the undiscovered country. But it tends to be one that people believe they can understand based on what has happened in the past. This is the reason people invest with successful money managers, and why every prospectus carries the disclaimer that "past experience does not ensure future performance." It is also why learned (and not so learned) disquisitions on innovation and innovation policy are so incomplete and unsatisfying — innovation like the future comes from where we least expect it, both in substance and the people who turn out to be the innovators. As Niels Bohr said, "[p]rediction is very difficult, especially about the future."
 But one thing that is predictable is that people will modify their behavior (at least to some extent) to adapt to changing circumstances. One such changing circumstance is the biosimilars provisions of the omnibus health care reform bill enacted into law almost three years ago. As has been discussed here and elsewhere extensively (and won't be repeated here), there are two general provisions to the law: the requirements and procedures for a biosimilar applicant to be granted approval of a biosimilar application (section 7002) and the provisions for litigation between the biosimilar applicant and the reference product sponsor (section 7003). The Food and Drug Administration is charged in large part with determining the requirements and procedures specified broadly by Congress in section 7002. The agency has hinted that it will propose rulemaking and issue guidelines by the end of the year, and these guidelines will not only establish the parameters of the biosimilar pathway but also affect how likely it will be that biosimilar drug manufacturers will apply for approval under the biosimilars provisions (codified under § 351(k) of the Public Health Service Act, codified at 42 U.S.C. § 262(k)) or under other sections (such as a full-blown Biologic License Application under § 505(a) of the Food, Drug and Cosmetics Act, codifed at 21 U.S.C. § 355(a)). These guidelines will also affect the likelihood that the biosimilar applicant will attempt to qualify for interchangeability and the incentives for producing so-called "bio-betters," i.e., biologic molecules having differences in structure and different (preferably improved) biological properties.
But one thing that is predictable is that people will modify their behavior (at least to some extent) to adapt to changing circumstances. One such changing circumstance is the biosimilars provisions of the omnibus health care reform bill enacted into law almost three years ago. As has been discussed here and elsewhere extensively (and won't be repeated here), there are two general provisions to the law: the requirements and procedures for a biosimilar applicant to be granted approval of a biosimilar application (section 7002) and the provisions for litigation between the biosimilar applicant and the reference product sponsor (section 7003). The Food and Drug Administration is charged in large part with determining the requirements and procedures specified broadly by Congress in section 7002. The agency has hinted that it will propose rulemaking and issue guidelines by the end of the year, and these guidelines will not only establish the parameters of the biosimilar pathway but also affect how likely it will be that biosimilar drug manufacturers will apply for approval under the biosimilars provisions (codified under § 351(k) of the Public Health Service Act, codified at 42 U.S.C. § 262(k)) or under other sections (such as a full-blown Biologic License Application under § 505(a) of the Food, Drug and Cosmetics Act, codifed at 21 U.S.C. § 355(a)). These guidelines will also affect the likelihood that the biosimilar applicant will attempt to qualify for interchangeability and the incentives for producing so-called "bio-betters," i.e., biologic molecules having differences in structure and different (preferably improved) biological properties.
The ligation provisions, on the other hand, depend on the existence of patents to protect biologic drugs after expiration of the twelve-year data exclusivity period. Superficially, these provisions are modeled on the Hatch-Waxman regime, insofar as the statute guides how patent infringement litigation will proceed. Superficially is where the resemblance to Hatch-Waxman ends, of course; a significant difference is the absence of an "Orange Book" defining what patents are to be the subject of litigation. In its stead is a complex negotiation protocol by which the parties decide which patents will be the subject of infringement litigation triggered by the filing of a biosimilar application. There has been speculation that the statute was intended to discourage litigation and its labyrinthine, almost Byzantine provisions support this view. It is likely to take at least five years to almost a decade before there is any case law developed enough to test the effectiveness of these provisions.
There is an alternative, however, that may tend to change unpredictably the course of biosimilar development. That alternative is for biologic drug innovators to eschew patenting altogether and thus avoid the possibility of protracted patent infringement litigation under the statute. There are a number of vulnerabilities to patents that claim biologic drugs that are not shared with small molecule drugs and that may make such an approach attractive. Most of these vulnerabilities stem from the greater complexity of biologic drugs; as shown below, biologic drugs have 2-3 orders of magnitude more atoms than small molecule drugs. In addition, biologic drugs are made (generally) in genetically engineered cells that impose their own variabilities (in post-translation modifications such as glycosylation for example) on the processes used to make such drugs. Further, the complexity makes the scope of patent protection on biologic drugs to be less certain than for small molecules, since even small differences in structure (such as the single methylene group that distinguishes valine from isoleucine) may have unpredictable effects on biologic drug structure, biological activity and immunogenicity. As a consequence, broad coverage for even structurally similar species of the same biologic drug are infrequently granted, and even when obtained may be of dubious provenance and reliability.
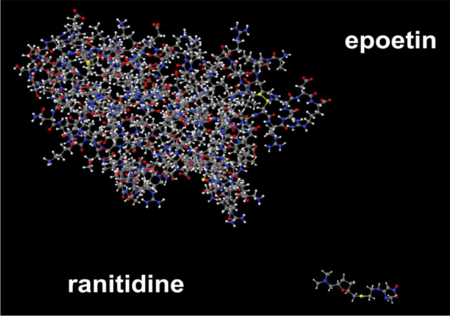
But this very complexity might be a source of an advantage for a biologic drug innovator not to pursue patent protection. In that case, the incentives for the innovator would be to provide as little information as required by the FDA for biologic drug approval. This would of necessity involve disclosure of how the biologic drug was made and its characterization, but would not include disclosure of the cell itself. Even if the disclosure to the agency included a detailed description of the cells, the transgenes and the methods and conditions for producing the recombinant cells, optimization of the particular cell line should not need to be revealed. And the uncertainty that would produce would provide a clear obstacle to a biosimilar manufacturer that might be a disincentive to trying to readily (i.e., predictably) produce a biosimilar version of a biologic drug.
The benefits of this approach for biologic drug innovators are evident: producing obstacles to the biosimilar applicant (for whom it would be more difficult to produce a biosimilar drug) as well as avoiding biosimilars litigation under the statute. It is unlikely that the Congressional staffers and their patrons intended this result. It is equally unlikely that the promised benefits of biosimilar availability (and the expected massive cost savings, especially to the government) will be achieved were innovators to eschew patenting. But this is yet another consequence of (mis)applying our experience with small molecule drugs and the Hatch-Waxman regime to biosimilars, and another example of the past not reliably informing the future.

Leave a comment