By Kevin E. Noonan —
 Under the Medicare Prescription Drug, Improvement and Modernization Act of 2003, settlements between innovator drug companies and generic competitors must be filed with the Federal Trade Commission (FTC). On May 3rd, the Commission released a compilation of the statistics relating to these settlements. Consistent with its long-time concern with and vendetta against settlements between innovator and generic drug companies, the Report highlights what it views as an increase of settlements where a generic company is compensated for staying off the market for longer than would be the case if it won ANDA litigation against the innovator. Viewing the same data a different way suggests that the frequency of such "pay-for-delay" agreements are in fact decreasing (while at the same time suggesting that the wastefulness associated with ANDA litigation is beginning to be appreciated by innovators and generic drug makers alike).
Under the Medicare Prescription Drug, Improvement and Modernization Act of 2003, settlements between innovator drug companies and generic competitors must be filed with the Federal Trade Commission (FTC). On May 3rd, the Commission released a compilation of the statistics relating to these settlements. Consistent with its long-time concern with and vendetta against settlements between innovator and generic drug companies, the Report highlights what it views as an increase of settlements where a generic company is compensated for staying off the market for longer than would be the case if it won ANDA litigation against the innovator. Viewing the same data a different way suggests that the frequency of such "pay-for-delay" agreements are in fact decreasing (while at the same time suggesting that the wastefulness associated with ANDA litigation is beginning to be appreciated by innovators and generic drug makers alike).
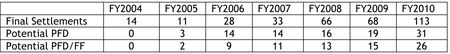
First, the stats. For the 2010 fiscal year (October 1, 2009 to September 20, 2010), there were 113 "final resolutions of patent disputes" between innovator and generic drugmakers (in the context of ANDA litigation). Thirty-one of these settlements involved both "compensation" to the generic manufacturer and an agreement that the generic drugmaker not market its product for a term longer than would have resulted from successful ANDA litigation. These settlements involved drugs having a combined U.S. sales of about $9.3 billion. In another 66 settlements, the FTC stated that there was a restriction on the ability of a generic drugmaker to sell generic versions of a drug, without any "explicit compensation." A little more than one half (36) of these settlements were with generic drugmakers who were not the first ANDA filers (and thus were not eligible for the 180-day exclusivity for a first ANDA filer under the Hatch-Waxman Act). The Report recognizes that these settlements involved "restrictions on entry in exchange for the ability to market the relevant product for some period prior to patent expiration" (i.e., earlier than the generic drugmaker would have been able to market its product if unsuccessful in challenging Orange Book-listed patents). Finally, a total of 16 settlements had no restrictions on market entry for the generic drugmaker.
All of the settlements between innovators and first ANDA filers had restrictions on time of market entry, and about half (26/49) also had compensation from the innovator to the generic drugmaker for market entry delay.
Comparing fiscal year 2010 with prior years, the Commission's Report states that it witnessed:
[A] significant increase in the number of final settlement agreements filed, as well as the number of settlements potentially involving pay-for-delay. The number of final settlements filed in FY 2010 is almost double the amount received in any previous year. Similarly, the number of potential pay-for-delay (PFD) settlements and the number of potential pay-for-delay settlements involving first filers (FF) substantially increased over any previous year.
These statements were supported by the following table:
A more expanded table might have been more informative:
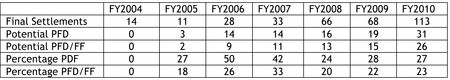
These numbers suggest that, while the absolute number of settlements having either market entry restrictions, compensation, or both have increased, this increase must be considered in the context of the overall increase in ANDA litigation settlements. Whether due to the scrutiny occasioned by FTC displeasure over the practice, or because of the threat of antitrust litigation (from state as well as private "attorneys general"; see "Second Circuit Denies En Banc Reconsideration in Cipro® Case"), "reverse settlement" agreements ("pay-for-delay" in FTC parlance) appear to be becoming less popular. This trend is essentially the same for first filers as well as other ANDA filers, and is not significantly more pronounced for first filers, who stand to lose the 180-day exclusivity benefit if they settle ANDA litigation.
The Report is accompanied by copies of settlements over Humira® (between Centocor and Abbott Labs), fentanyl (Cephalon and Watson), and gemcitabine (Eli Lilly & Co. against a number of foreign generic drug companies), among others. While the trend towards settling ANDA cases seems to support those who argue, inter alia, that the Hatch-Waxman ANDA regime is wasteful of resource that might better be spent addressing deficiencies in the pharmaceutical pipeline (see "Maybe Hatch-Waxman Data Exclusivity Isn't So Good For Traditional Drugs After All"), within that trend is a declining likelihood to settle ANDA litigation with agreements that provide monetary compensation to generic drugmakers in exchange for delayed market entry. This trend should be more welcome to the FTC than the latest Report seems to indicate.

Leave a comment